
Bringing color to water treatment
August 08, 2018 0 Comments
How can a cooling tower spread Legionnaires' Disease?
January 29, 2018 0 Comments

Brewing up success!
January 10, 2018 0 Comments
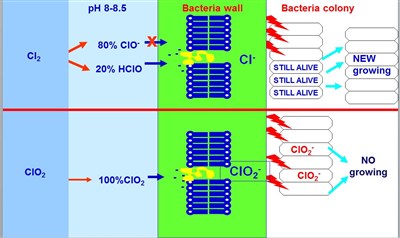
Chlorine vs. Chlorine Dioxide different features against biofilm growth
November 28, 2017 0 Comments

Sulfites for Oxygen Control
November 07, 2017 0 Comments








