
How To Prepare For Reopening Your Pool Facility
April 20, 2021 0 Comments
Does Chlorine kill COVID ?
April 20, 2021 0 Comments

Do water softeners remove chlorine?
April 12, 2021 0 Comments

Free Chlorine and DPD test!
March 22, 2021 0 Comments
Peracetic acid - the new hero in hospitals
February 08, 2021 0 Comments

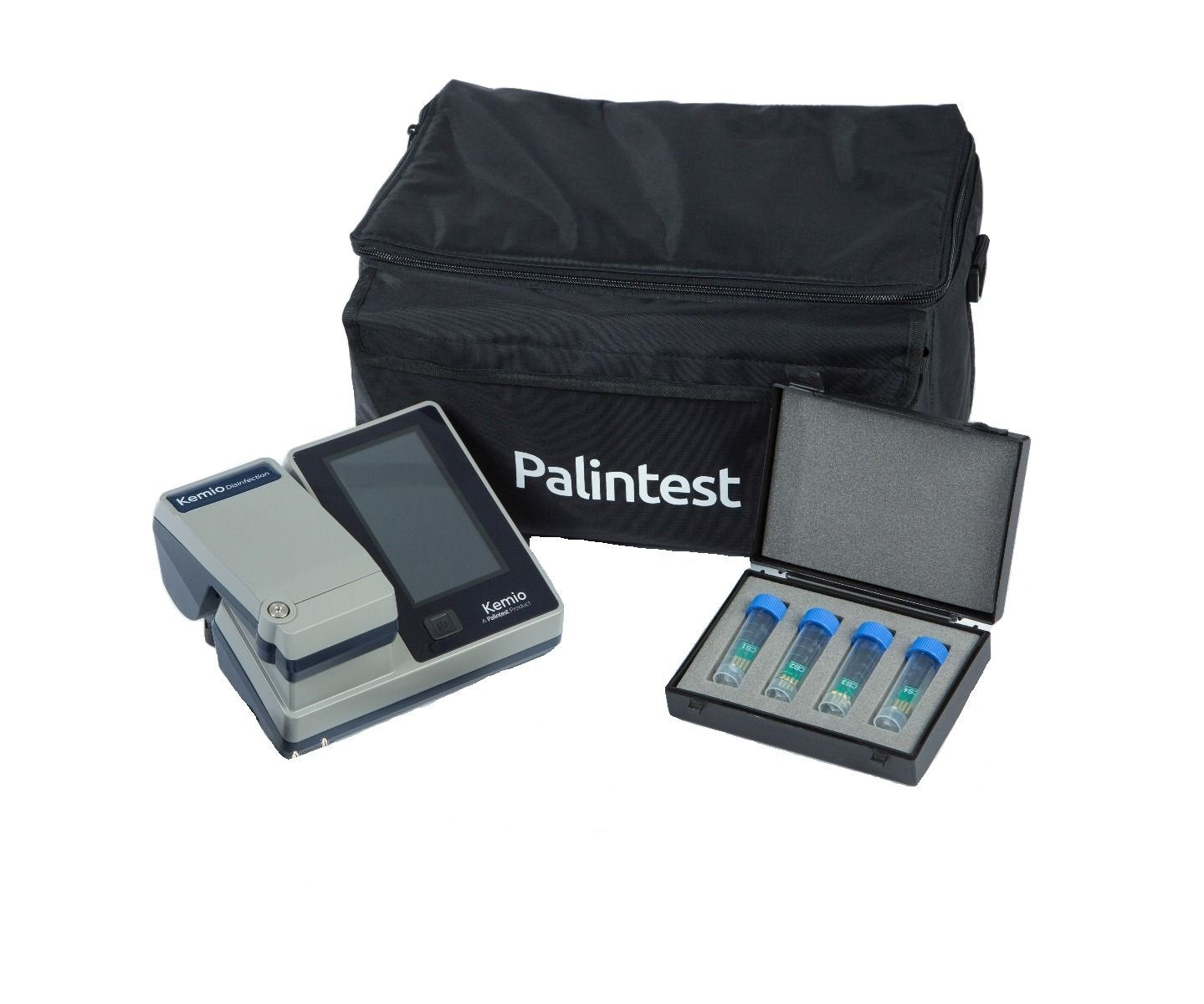
Kemio help assure correct disinfection for food, beverage and containers.
April 14, 2020 0 Comments
Kemio™ is the next generation measurement platform, delivering the validation you need from a method you can trust. Kemio™ Disinfection enables expert disinfection validation, using an EPA approved method.
- Chlorine, Chlorine Dioxide, Chlorite and PAA (Peracetic acid) testing on one instrument
- Get clear pass/fail results for confident decision making
- Prompt immediate action, generating test results on site in 60 seconds
- A truly simple test method, Kemio requires no user training.
- Automatically stores all results for a complete, auditable dataset
- Suitable for all sample types, Kemio is not affected by colour, turbidity, floating particles or ambient light

Kemio, the best instrument for disinfection monitoring!
October 22, 2019 0 Comments
How can a cooling tower spread Legionnaires' Disease?
January 29, 2018 0 Comments

Brewing up success!
January 10, 2018 0 Comments
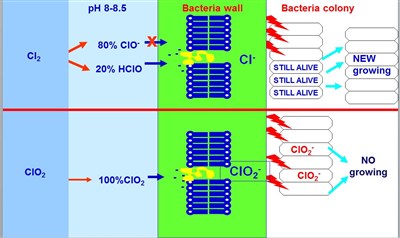
Chlorine vs. Chlorine Dioxide different features against biofilm growth
November 28, 2017 0 Comments



