Boiler Water Conductivity Measurement Fundamentals
February 24, 2016 0 Comments
As a boiler generates steam, any impurities which are in the boiler feedwater and which do not boil off with the steam will concentrate in the boiler water.

Inside the boiler the heat generates steam bubbles inside the water. These bubbles float and burst when they reach the water surface, releasing the steam. As the dissolved solids become more and more concentrated, the steam bubbles tend to become more stable, failing to burst as they reach the water surface of the boiler. There comes a point (depending on boiler pressure, size, and steam load) where a substantial part of the steam space in the boiler becomes filled with bubbles and foam is carried over into the steam main.
This is undesirable not only because the steam is excessively wet as it leaves the boiler, but it contains boiler water with a high level of dissolved and perhaps suspended solids. These solids will contaminate control valves, heat exchangers and steam traps, impacting the performance and operation of the system.
There are several causes for whilst foaming; high levels of suspended solids, high alkalinity or contamination by oils and fats but, the most common cause of carryover (provided these other factors are properly controlled) is a high Total Dissolved Solids (TDS) level. Careful control of boiler water’s TDS level, together with attention to these other factors should ensure that the risk of foaming and carryover are minimized.
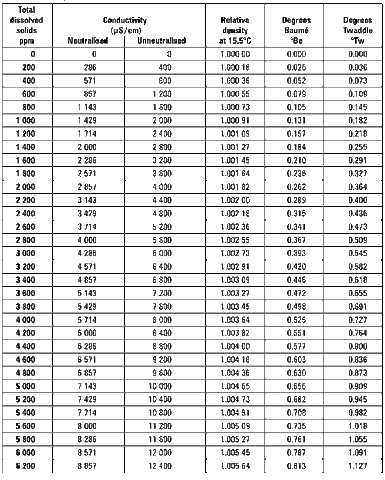
TDS may be expressed in a number of different units, and following table gives some approximate conversions from TDS in ppm to other units.
Degrees Baumé and degrees Twaddle (also spelt Twaddell) are alternative hydrometer scales.
Water Total Dissolved Solids can is determining by measuring the water conductivity level. Pure water does not conduct electricity. However, once different salts dissolve in the water, they start conducting electricity. The more dissolved salts, the higher the conductivity. To learn more, just click here!
Boiler water sampling
The boiler water TDS may be measured either by; Taking a sample, and determining the TDS external to the boiler, or by a sensor inside the boiler providing a signal to an external monitor.
Need a portable TDS meter? Click Here!
Sampling for external analysis
When taking a sample of boiler water it is important to ensure that it is representative. It is not recommended that the sample be taken from level gauge glasses or external control chambers; the water here is relatively pure condensate formed by the continual condensation of steam in the external glass / chamber. Similarly, samples from close to the boiler feedwater inlet connection are likely to give a false reading.
Nowadays, most boilermakers install a connection for TDS blowdown, and it is generally possible to obtain a representative sample from this location.
If water is simply drawn from the boiler, a proportion will violently flash to steam as its pressure is reduced. Not only is this potentially very dangerous to the operator, but any subsequent analysis will also be quite wrong, due to the loss of the flash steam concentrating the sample.
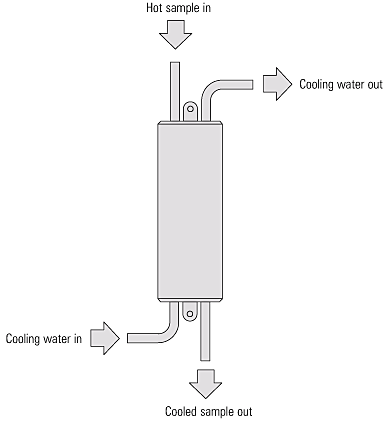
Since a cool sample is required for analysis, a sample cooler will also save considerable time and encourage more frequent testing.
A sample cooler is a small heat exchanger that uses cold mains water to cool the blowdown water sample.
Do you need a Sample Cooler? Click Here Now!
Conductivity method
The electrical conductivity of water also depends on the type and amount of dissolved solids contained. Since acidity and alkalinity have a large effect on the electrical conductivity, it is necessary to neutralize the sample of boiler water before measuring its conductivity. The procedure is as follows:
- Add a few drops of phenolphthalein indicator solution to the cooled sample (< 25°C).
- If the sample is alkaline, a strong purple color is obtained.
- Add acetic acid (typically 5%) drop by drop to neutralize the sample, mixing until the color disappears.
Example
Conductivity of a neutralized sample at 25 °C = 5,000 μS/cm
TDS = 5,000x0.7μS/cm
TDS = 3,500 ppm
Alternatively, the battery powered, temperature compensated conductivity meter shown next is suitable for use up to a temperature of 45°C. It is necessary to measure the conductivity of the boiler water inside the boiler or in the blowdown line. Obviously, the conditions are very different from those of the sample obtained via the sample cooler which will be cooled and subsequently neutralized (pH = 7). The main aspects are the great temperature difference and high pH. An increase in temperature results in an increase in electrical conductivity. For boiler water, the conductivity increases at the rate of approximately 2% (of the value at 25°C) for every 1°C increase in temperature. This can be written as:
σT = σ25 [1 + α (T – 25)] σT = Conductivity at temperature T (µS / cm)
α = Temperature coefficient, per °C (Typically 0.02 / °C or 2%°C)
T = Temperature (°C)
A boiler water sample has an un-neutralized conductivity of 5 000 µS / cm at 25°C. What is the conductivity of the boiler water at 10 bar g?At 10 bar g, saturation temperature = 184 °C (from steam tables)
σT = 5,000 [1+0.02(184-25)]
This means that the effects of the temperature have to be allowed for in the blowdown controller, either by automatic temperature compensation, or by assuming that the boiler pressure (and hence temperature) is constant. The small variations in boiler pressure during load variations have only a relatively small effect, but if accurate TDS readings are required on boilers which are operated at widely varying pressures then automatic temperature compensation is essential.
Cell constant
Where: K = Cell constant (cm)
From the previous example the boiler water conductivity was 20 900 µS / cm. For a cell constant of 0.3, what is the resistance measured by the controller?
Resistance = 14.4 Ohm
It is therefore necessary to use an ac voltage to measure the probe resistance and this is the method always to be preferred in blowdown controllers. A relatively high frequency (for example 1 000 Hz) is necessary to avoid polarization at the high conductivities of boiler water.
Whilst the boiler water conductivity is converted to a resistance through the probe, it cannot be measured using a simple dc resistance meter. If a dc voltage is applied to the probe, tiny hydrogen or oxygen bubbles are formed on the surface due to electrolysis of the water. This effect, called electrolytic polarization, causes a much higher resistance to be measured.
σ = Conductivity (S/cm)
R = Resistance ( Ohms)
Conductivity and resistance are related by the cell constant, as seen in the following equation:
The further the probe tip is from any part of the boiler, the higher the cell constant. Any differences in cell constant are taken into consideration when 'calibrating' the controller.
A probe used to measure the conductivity of a liquid has a 'cell constant'. The value of this constant depends on the physical layout of the probe and the electrical path through the liquid.
σT = 20,900 µS/cm
σ25 = Conductivity at 25 °C (µS / cm)
Where:
Conductivity measurement in the boiler
TDS = 3,500 ppm
Conductivity of a neutralized sample at 25 °C = 5,000 μS/cm
Note: This relationship (shown in 3.12.2) is only valid for a neutral sample at 25°C.
TDS (ppm) = (conductivity in μS/cm) x 0.7
The TDS in ppm is then approximately as shown in the equation:
Where:
R = Resistance ( Ohms)
K = Cell constant (cm)
σ = Conductivity (S/cm)
From the previous example the boiler water conductivity was 20 900 µS / cm. For a cell constant of 0.3, what is the resistance measured by the controller?
Resistance = 14.4 Ohm
Need a Boiler TDS Controller? Go Here Now!
Also in Blog

Advanced Cooling Tower Management: Enhancing Efficiency with Lakewood Model 140
February 28, 2024 0 Comments

Optimizing Cooling Tower Performance: Understanding Efficiency, Maintenance, and Water Quality Management
February 28, 2024 0 Comments

Revolutionizing Water Analysis: Everything You Need to Know About the Kemio KEM10DIS
April 19, 2023 0 Comments




