
Avoid this issues when starting a cooling tower
March 20, 2023 0 Comments

How does a cooling tower work ?
April 15, 2021 0 Comments

How does a cooling tower work ?
March 02, 2021 0 Comments

How does the cooling tower startup work?
March 01, 2021 0 Comments
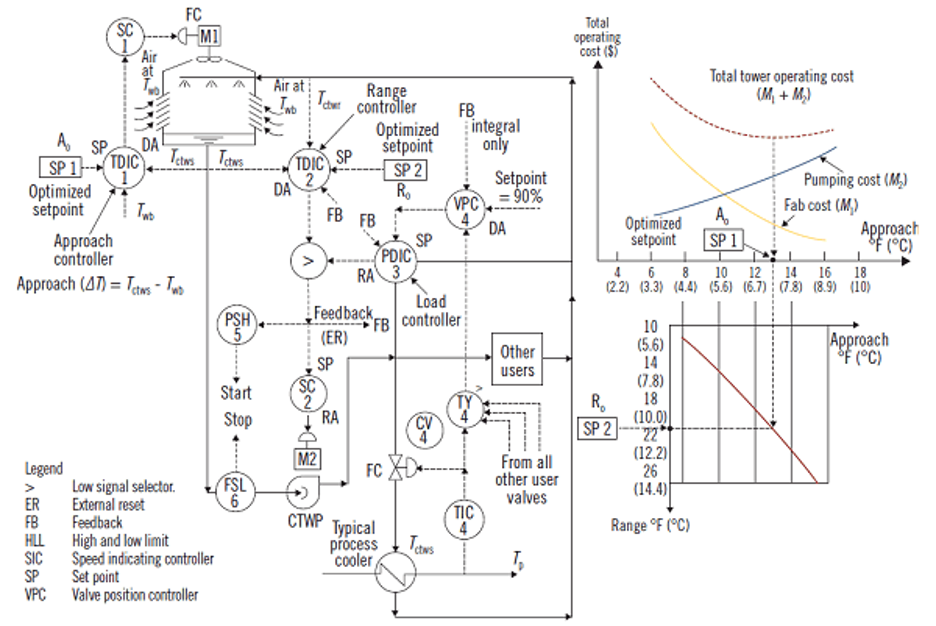
Optimization of cooling towers
June 23, 2020 0 Comments
The load on a cooling tower depends on the flow and temperature of the water returning from the process. The controlled variable is the temperature of the cooling water that is sent back to the process and the manipulated variable is the air flow through the tower, which can be changed either by adjusting the speed of variable-speed fans or by starting and stopping a number of constant-speed fans.

Bringing color to water treatment
August 08, 2018 0 Comments
How can a cooling tower spread Legionnaires' Disease?
January 29, 2018 0 Comments






