
Avoid this issues when starting a cooling tower
March 20, 2023 0 Comments
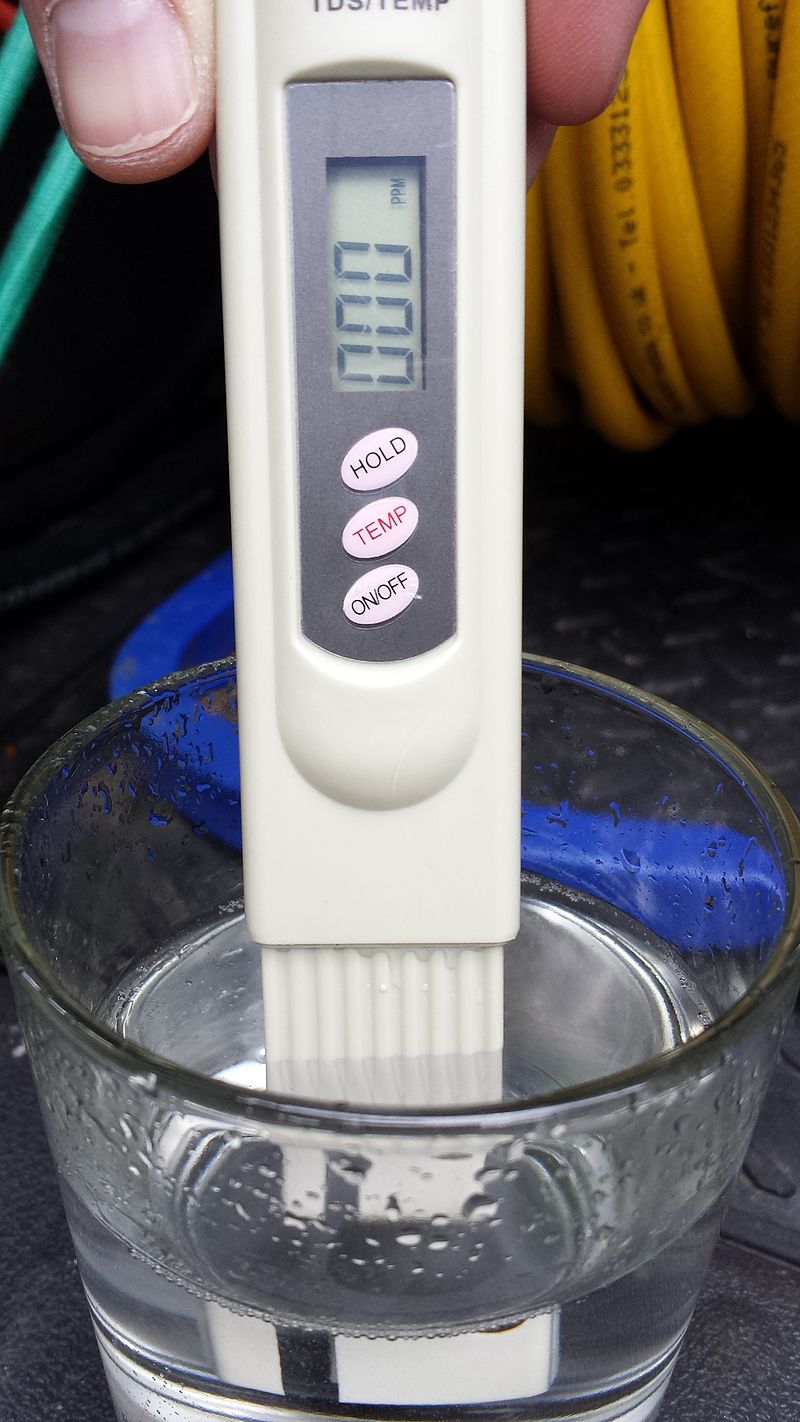
What is water TDS?
May 06, 2021 0 Comments

Boiling point of water
March 10, 2021 0 Comments

Sulfites for Oxygen Control
November 07, 2017 0 Comments

Reduccion del uso de agua en torres de enfriamiento con automatización
September 12, 2017 0 Comments
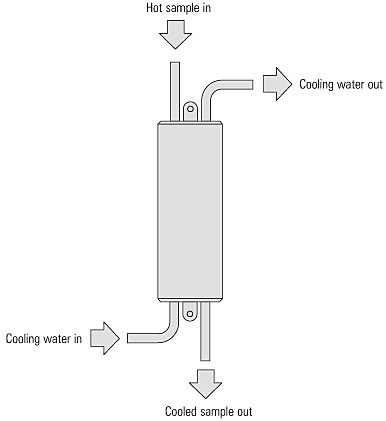
Fundamentos de la Medicion de la Conductividad de Calderas
March 15, 2016 0 Comments







